Description
Assure EcoTest Covid-19 Test - 20/box
This test is not intended for at home use:
- This test has not been FDA cleared or approved;
- This test has been authorized by FDA under an EUA for use by authorized laboratories;
- This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens; and
- This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.
- The COVID-19 Antibody Rapid test will detect antibodies 5 to 7 days after symptoms first appear
- The test will show clear results in 15 minutes, unlike a PCR test, which can take hours
- The COVID-19 Antibody Rapid Test is cost effective, at approx. 5% of the cost of a PCR test
- The test is CLIA Waived
Assure Letter of Authorization
Instructions for Use
Antibody Fact Sheet for Healthcare Professionals
Antibody Fact Sheet for Patients

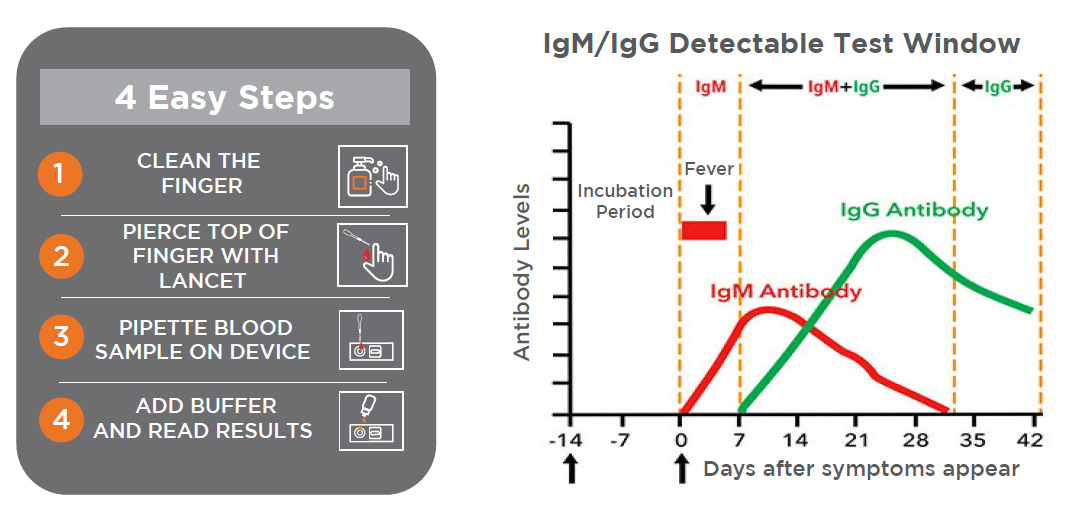
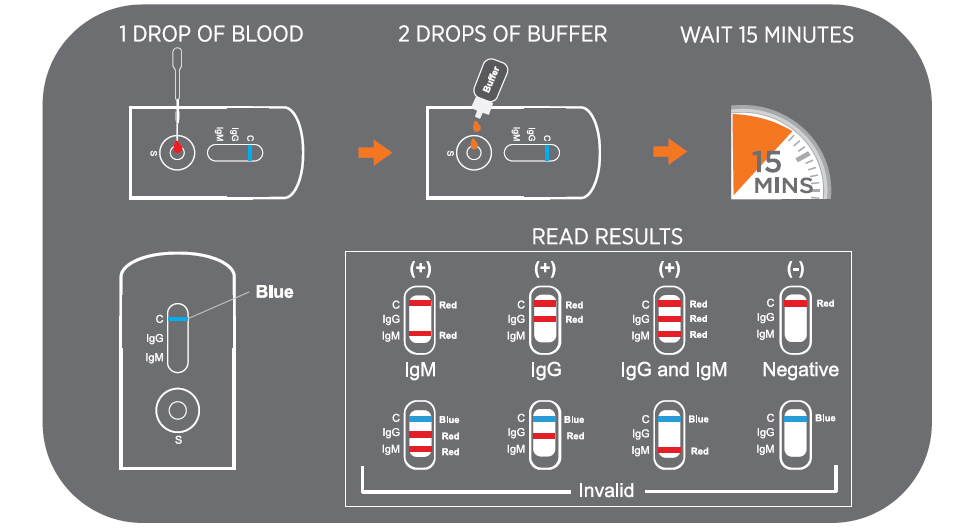
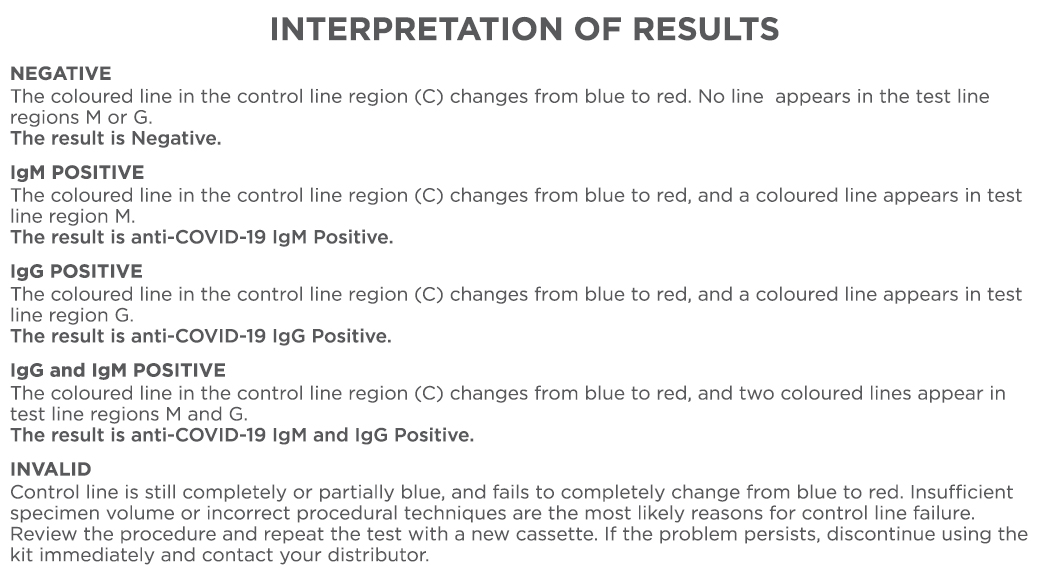
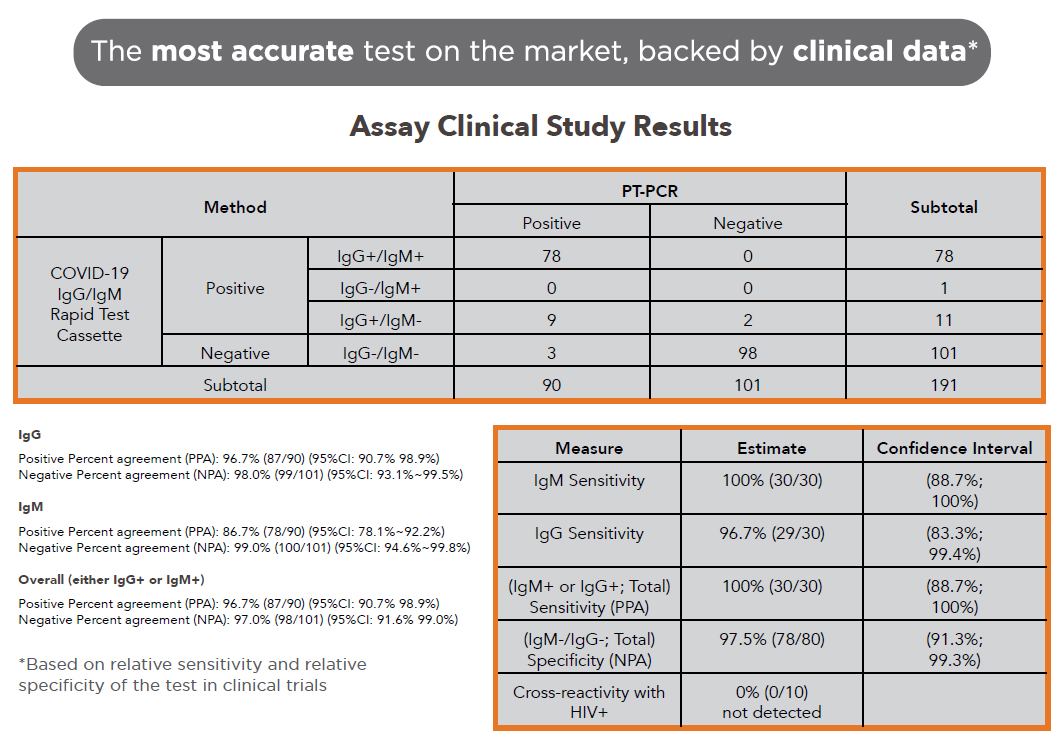
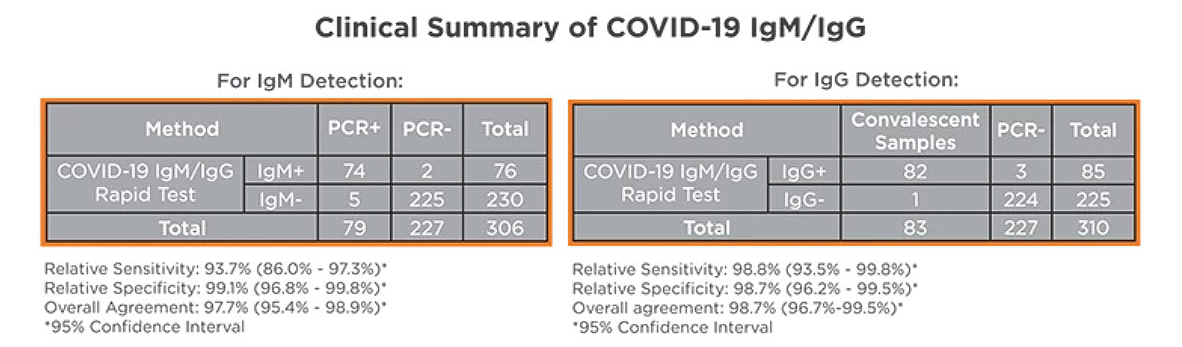
CLINICAL DATA
SARS-CoV-2 Antigen and IgM/IgG Antibody Test Results and Clinical Significance

• This test has not been FDA cleared or approved;
• This test has been authorized by FDA under an EUA for use by authorized laboratories;
• This test has been authorized only for the presence of IgM and IgG antibodies against SARS-CoV-2, not for any other viruses or pathogens; and
• This test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner




