Description
AVAILABILITY DATE FOR THIS ITEM IS TBD
PLEASE TAKE A LOOK AT OUR OTHER COVID-19 ANTIGEN TEST KIT:
https://www.e-medtek.com/clarity-diagnostics-covid-19-antigen-rapid-test-25-box/
FREE SHIPPING (UPS Ground only)
For faster shipping, please call our office at 801-561-3339.
CareStart COVID-19 Rapid Antigen POC Test - 20ct
CLIA Certificate of Waiver required to purchase this product.
This test has not been FDA cleared or approved; the test has been authorized by FDA under an Emergency Use Authorization (EUA) for use by laboratories certified under the CLIA that meet the requirements to perform moderate, high or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
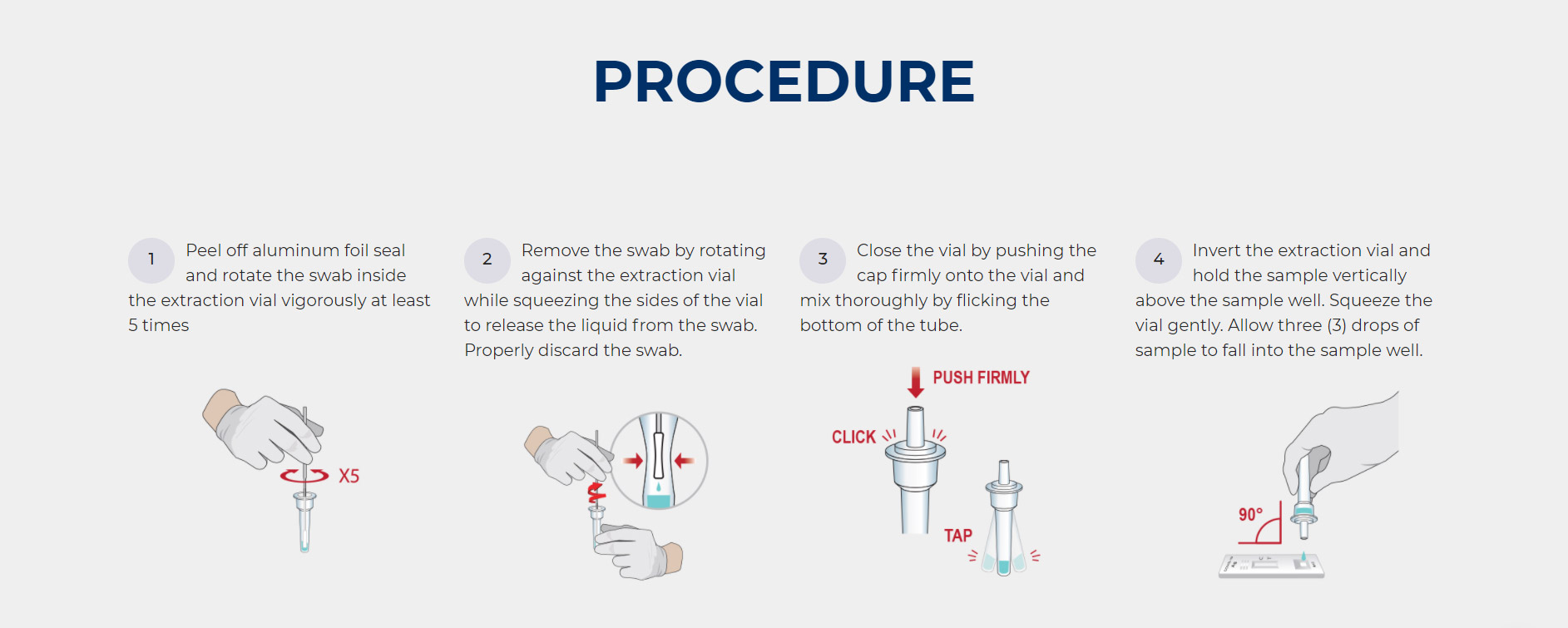
The CareStart™ COVID-19 Rapid Antigen Test is a lateral flow immunochromatographic assay for the detection of extracted nucleocapsid protein antigens specific to SARS-CoV-2 in swab specimens directly collected from individuals who are suspected of COVID-19 by their healthcare providers.
One of the benefits of buying rapid antigen tests is that they provide rapid results, which means they could be done in emergency rooms or doctor’s offices to quickly determine whether someone is sick with Covid-19. "The technology behind it is a real game changer," said former FDA commissioner Scott Gottlieb. As an intended point-of-care (POC) designated test performed by medical professionals at a 10-minute processing time, the CareStart™ COVID-19 Rapid Antigen Test helps provide critical answers about active infections to patients and healthcare workers alike.
- Rapid results at 10 minutes
- No lab equipment or additional instrument required
- Minimally invasive specimen collection (anterior nasal swab)
- Intended at POC setting (i.e., in patient care settings) by medical professional with CLIA Certificate of Waiver
- Detects SARS-CoV-2 nucleocapsid protein antigen via a lateral flow assay
- Identify acute infection with high sensitivity and 100% specificity
Includes:
- 20 Test devices
- 20 Assay buffer
- 20 Extraction vials and caps
- 20 Specimen collection swabs
- 1 Positive and 1 negative
- 1 Instructions for Use
FDA Emergency Use Authorization
Fact Sheet for Healthcare Professionals
COMMON QUESTIONS:
What are COVID-19 antigen tests?
Antigen tests are used for diagnosing respiratory pathogens. These include influenza viruses and respiratory syncytial virus. The FDA has granted emergency use authorization (EUA) for rapid antigen tests that can correctly identify SARS-CoV-2.
How is a COVID-19 test performed?
A POC diagnostic test (molecular or antigen) uses a mucus sample from the nose or throat. It can be analyzed at the doctor’s office or clinic where the sample is collected and the result may be available in minutes.
How long do COVID-19 antigen tests take?
Some antigen tests can return results at 10 minutes, thus they are frequently called "rapid antigen tests."
How accurate is a COVID-19 rapid antigen test?
Positive results are usually very accurate, although false positives can happen, especially in locations where very few people have the virus. Negative results may need to be confirmed with a molecular test.
What is the difference between COVID-19 antigen and PCR tests?
PCR tests detect viral RNA, and you may wait several days for test results. Antigen tests (rapid diagnostic tests) detect specific proteins on the surface of the coronavirus, and test results may come back in as little as 10 minutes.
What are the benefits when you buy rapid antigen tests from MedTek?
- Excellent customer service
- Price-matched pricing
- Free shipping
If you would like to order a pallet (640 boxes)
call (801) 561-3339 for a price quote.
REFUND POLICY for COVID-19 Test: Our standard refund policy does not apply when buying COVID-19 rapid tests. All sales are final once goods are shipped and in transit with courier. NO EXCHANGES OR REFUNDS WILL BE MADE.
The CareStartTM COVID-19 Antigen test has not been FDA cleared or approved. This test has been authorized by FDA under an EUA for use by authorized laboratories and at the Point of Care by medical professionals operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation. This test has been authorized only to detect the presence of the SARS-CoV-2 nucleocapsid protein antigen, not for any other viruses or pathogens; this test is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Act, 21 U.S.C. § 360bbb-3(b) (1), unless the authorization is terminated or revoked sooner.





